Vapour Pressure of Liquid Solutions
Vapour Pressure of Liquid Solutions: Overview
This Topic covers sub-topics such as Boiling Point, Vapour Pressure, Freezing Point, Saturated Vapour Pressure, Partial Vapour Pressure, Variation of Vapour Pressure with Temperature and, Standard Boiling Point
Important Questions on Vapour Pressure of Liquid Solutions
Assertion: When is added to water a depression in the freezing point is observed.
Reason: The lowering of the vapour pressure of a solution causes depression in the freezing point.
What is the difference between vapour pressure and saturated vapour pressure?
Which of the following solutions does not show positive deviation?
How does vapour pressure relates to partial vapour pressure?
What is the difference between vapour pressure and partial vapour pressure?
Define partial vapour pressure.
Standard boiling point of a liquid is higher than the normal boiling point.
State why pressure cooker is used to cook food?
Define standard boiling point.
Liquids at higher altitudes boils at a lower temperature.
Vapour that remains in contact with its liquid surface within a closed space is called _____.
(Choose from: saturated vapour/unsaturated vapour)
Methanol and ethanol form nearly ideal solutions at . A solution is made by mixing of Methanol and ethanol the partial pressure ethanol. Calculate the partial pressure of its constituents and the total pressure of the solution.
.
Define freezing point of liquid.
What is boiling point of liquid?
What are the factors which affect vapour pressure?
Explain the effect of temperature on the vapour pressure of a liquid.
What is a vapour pressure of liquid?
Vapour pressure of a liquid increases with
A person living at high altitude region observed that cooking food without using pressure cooker takes more time. The reason for this observation is that
The pair of boiling point and compound are given as,
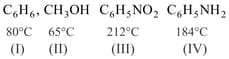
Which will show lowest vapour pressure at room temperature?
